Find Bio-Tech’s NIPT Received Wonderful Result in EQA
Time Published:2017-07-04Source:Author:
Browse:0 Print
Font Size:LargeMediumSmall
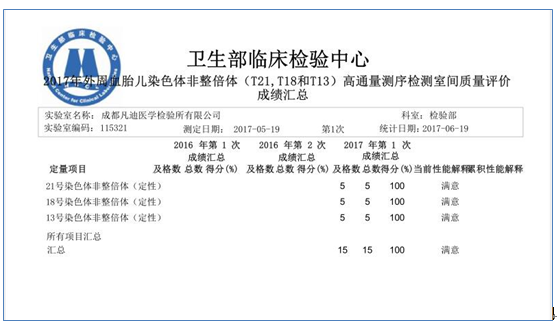
In June 2017, National Center for Clinical Laboratories
of the Ministry of Health issued the EQA Report on the first-time national PB
fetal chromosome aneuploid (T21, T18 and T13) high-throughput sequencing.
Chengdu Find Bio-Tech Co., Ltd. passed EQA by gaining 100 scores, which proves
that Find Bio-Tech has the ability to apply its high-throughput sequencing
technologies in clinical antenatal screening and diagnosis.
EQA uses comparisons among laboratories to
confirm laboratory ability. As a matter of fact, it refers to the verification
activity that evaluates, supervises and confirms the laboratory ability to
ensure they maintain high testing capabilities. By participating in EQA
program, it issues the evidence to evaluate whether the laboratory data are
reliable and valid. Since the Ministry of Health started the EQA of PB fetal
chromosome aneuploid (T21, T18 and T13) high-throughput sequencing in 2014, it
has improved the service quality of all such laboratories in China. After years
of making clinical sample testing and providing excellent service, Find
Bio-Tech now owns the complete, regulated and high-quality laboratory gene
testing techs, thus becoming a major force of in NIPT.
As high-throughput sequencing technology
develops rapidly and is put into clinical applications, Find Bio-Tech keeps
following national policies and regulations and works hard to push forward
development of gene testing. Taking the meticulous and responsible attitude,
Find Bio-Tech ensures the efficiency and accuracy of each sample in gene
testing, and is dedicated to promoting the safe, accurate and rapid NIPT,
genetic analysis of folic metabolism disorder, gene testing for single-gene
inheritance disease, PGS/PGD, chromosome abnormality testing, newborn
inheritance metabolism disease testing and other testing service related to
high-quality child birth and pregnancy for clinical use for the public. At the
same time, it also provides genetic testing services for individual medicines
such as cancer targeted drugs, tumor chemotherapeutics,
anti-high-blood-pressure medicines, anti-high-blood-sugar medicines, and
anti-high-blood-fat medicines.


